How to optimize your mobile phase to improve selectivity and resolution in chromatography
As previously discussed, the most efficient way you can improve resolution is to optimize selectivity. This parameter is most readily influenced by two factors, the stationary phase and the mobile phase. From selectivity triangles to gradient elution, this post is about how you can use the mobile phase to achieve the best possible selectivity and resolution in your separations.
There is nothing like some good pumpkin pie at this time of the year. Just like with any baking task, the importance of the ingredients cannot be underestimated. You have to pick the right type of flour and you should to choose the right type of sugar. Not everything will taste great with whole wheat flour. Sometimes you need the nutty brown sugar taste rather than just the normal sweet of the white sugar.
Just like choosing the right type of sugar and flour make all the difference in the final pie,
selecting the right stationary and mobile phases makes all the difference in achieving the best selectivity in your chromatography runs.
In the previous blog post, I gave some tips on how to establish the optimal stationary phase for your separations. Once you’ve accomplished this, you need to choose the mobile phase with the most suitable selectivity. For popular phases like Si or C18 this can be determined on a TLC plate.
In other words, you need to find the solvent or solvent mixture that separates the substance of interest with the greatest possible distance to the adjacent components.
In general, every solvent has its own selective properties. L.R. Snyder and J.J. Kirkland investigated and compared various solvents and grouped those with similar effects together into selectivity groups. The selectivity groups are organized into a selectivity triangle that makes it possible to visually compare solvents.
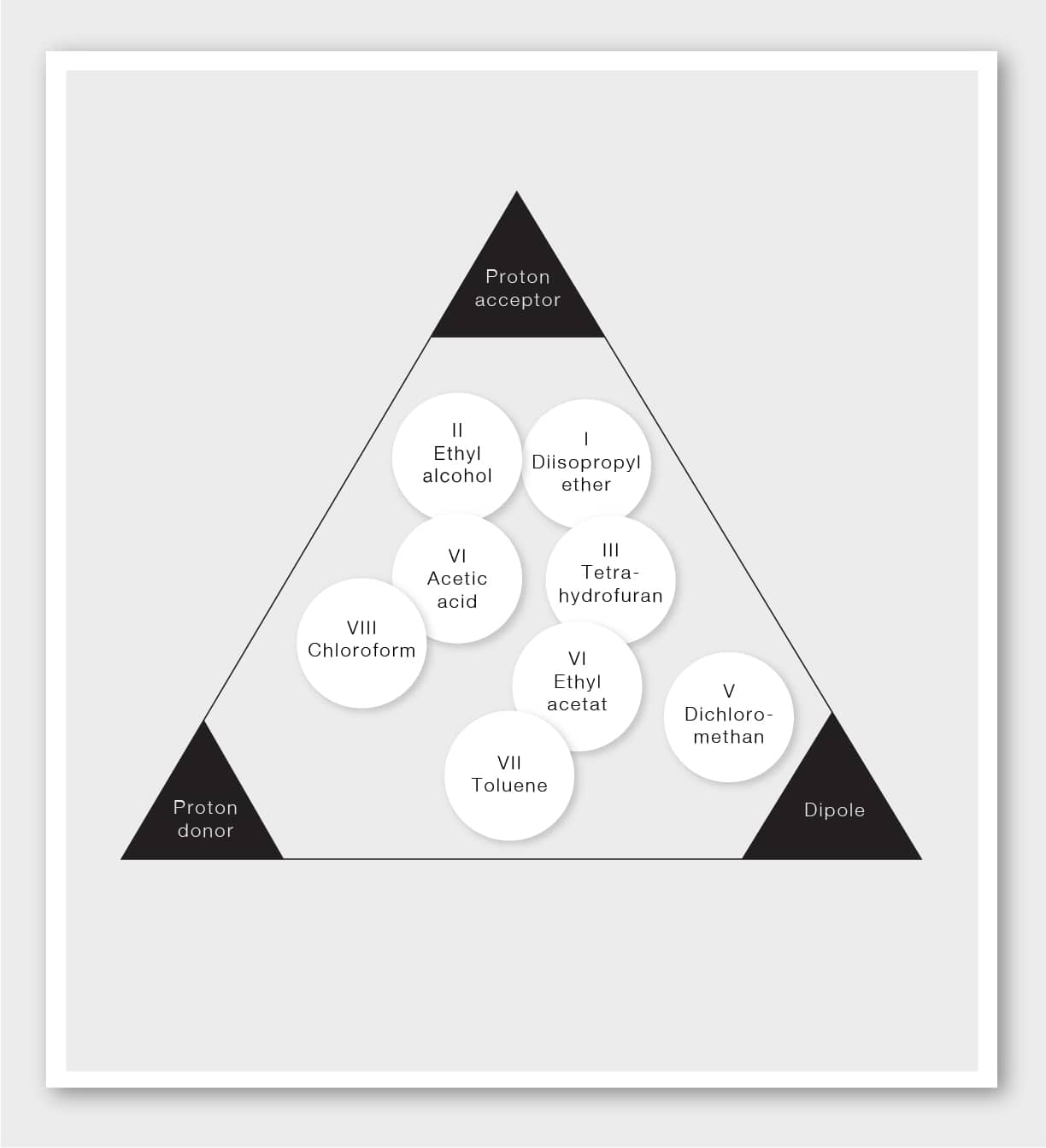
The most important practical consequence of the selectivity triangle is that if a certain solvent cannot provide sufficient selectivity in a given separation, it is unlikely that any other solvent in the same group can do so. Instead, you should use solvents from other selectivity groups.
Choosing a solvent from groups as far away from each other as possible on the triangle guarantees the highest difference in selectivity.
Most separations are performed with a combination of two solvents. In these situations, you should also establish the ratio between the two solvents.
Frequently, one starts an isocratic separation, i.e. the mobile phase composition remains constant throughout the entire separation process. In practice, an isocratic separation is often impossible to achieve. This is especially true when dealing with compounds whose polarity varies widely or the mixture to be purified consists of many components. Whenever the Rf values are too high, the analysis times are excessively prolonged. In separations where the Rf values are too low, the compounds could only be eluted from the column with a great deal of time and solvent.
To alleviate these effects, we may need to change the composition of the mobile phase over the course of the chromatographic run. With so-called gradient elution, you can vary the composition of the mobile phase by increasing the amount of solvent with higher eluting strength. Consequently, at the beginning of the run when the mobile phase strength is low, the analyte will be concentrated into the stationary phase at the head of the column. As the mobile phase strength increases, the analyte will begin to partition into the mobile phase and move along the column. At some point, the analyte may be wholly partitioned into the mobile phase and will be moving with the same velocity as the mobile phase.
As a result, by using gradient elution, the quality of the separation is improved and, as a positive side effect, the run time is reduced.
When optimizing the mobile phase, you should keep in mind another important characteristic, the ability of the mobile phase to completely solubilize the sample. Please bear in mind that solvent optimization should not compromise sample solubility.
By the way, when choosing the solvent you should also pay attention to other parameters, such as UV absorption, column pressure, solvent purity and solvent stability.
I hope I have shown you that altering the selectivity provides an excellent means of optimizing the chromatographic resolution. Small changes in selectivity can lead to large desirable changes in resolution.
But what about efficiency and retention? Rest assured they will not be forgotten. Just keep coming back to the blog so that you don’t miss out on any posts where I also address these two characteristics. And if you are still feeling hungry, check out my previous post on how I baked a cheesecake and pondered about “the golden mean” in flash chromatography.
Till next time,

WANT TO STAY IN TOUCH?
Click on the button and receive the latest posts directly in your messenger!
Related Posts
10th December 2018
How to choose a stationary phase, optimize selectivity and get better resolution in chromatography
See the effects of selectivity on separation quality and get some great tips on how to optimize resolution by finding the ideal stationary phase for your application→
22nd June 2018
World Cup Fever: How to Score Purer Compounds by Improving Resolution in Chromatography
Soccer fans or not, all chemists want to give impurities a red card. Read today how to improve resolution and score purer compounds →
7th February 2019
Three chromatography problems the new Pure system solves
There are challenges in chromatography when it comes to user, sample and environmental safety. See how the Pure chromatography system sets out to solve them →
8th May 2019
Putting safety first during the chromatography process
Bart discusses how to maximize the safety of user, sample and the surroundings during the chromatography process →








I find blog very helpful but some things still confuse me- So in RPC, mobile phase is polar but non-polar solvents have high strength. So what do we do? Use polar mobile phase to elute polar samples and then we change it to non-polar ones?
I have seen someone using mixture of methanol or ethanol(don’t remember) and hexane, so the mobile phase is kinda non-polar, so I assumed that it was NPC. is that right? Is NpC used to separate non-polar samples? If yes, why polar solvents(like water) is considered as strongest?
Thanks in advance.
Hi Nina!
Thanks for the comment.
In RPC the mobile phase is polar (mostly water and/or mixtures of water with polar (water miscible) solvents like acetonitrile, methanol,…). The stationary phase is non-polar (often C18 hydrocarbon chain).
The sample compounds partition between the mobile phase and the stationary phase. The more hydrophobic the compounds are, the more retained they will be on the stationary phase. The more polar they are, the more they will prefer the mobile phase.
The retention can be adjusted by modifying the composition of the mobile phase: a high concentration of organic solvent will reduce retention, a high water content will increase retention.
In NPC the mobile phase is non-polar (hexane or heptane or mixtures with solvents like ethylacetate, methanol etc). The stationary phase is polar.
The more polar the compounds are the more they will be retained on the stationary phase. The more hydrophobic they are the more they will prefer the mobile phase.
In Chromatography the main principle is “like for like”. This means polar compounds will retain best on a polar stationary phase.
We often see people use C18 for polar compounds which is not a good combination. To increase retention of polar compounds on a C18 phase the water content needs to increase. If retention is still not enough they only solution will be to switch to a more polar stationary phase (Silica, amina, diol)